Telephone:
+86 0417-3909999
Fax:
+86 0417-3909999
Contact:
Mr. Liu
website : en.ykblnc.com
company website:
www.ykblnc.com
E-mail:
bllzj888@163.com
address:
19-1 East, North Line, Yingda Road, Laobian District, Yingkou City, Liaoning Province
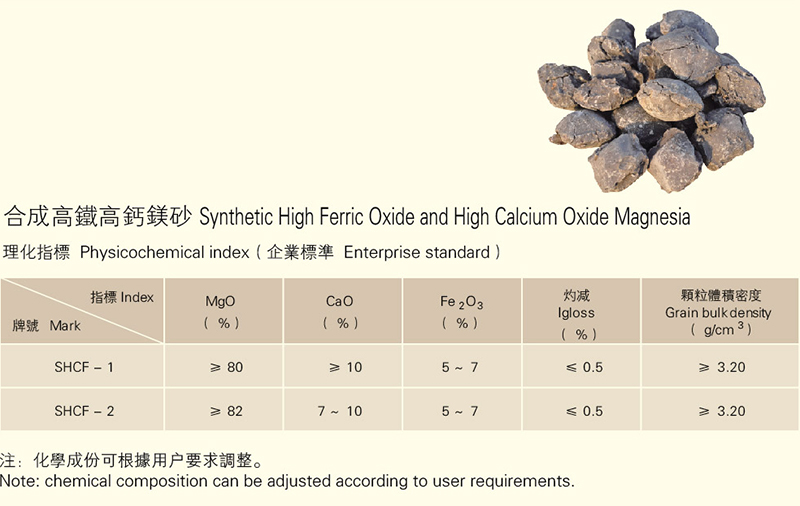
Magnesia can be divided into natural magnesia and seawater magnesia or synthetic magnesia. Natural magnesia mainly comes from natural magnesite, electric furnace magnesia carbon brick, but it is difficult to obtain high-purity and high-quality products due to the quality of raw ore. So far, seawater magnesia has become the main source of magnesia used in industrialized countries. The theoretical content of MgO in magnesite is 47.3%, and the remaining impurities are CaO, SiO2, iron, etc. The magnesite is heated in air to 700 ° C ~ 800 ° C decomposition, called "light burned magnesia", further heating When it is above 1530 ° C, the inactive periclase becomes hard-burned magnesia, which is called "dead burned magnesia". Synthetic seawater magnesia for industrial production is made from seawater and digested dolomite. The precipitated Mg(OH)2 is calcined to obtain MgO, and the ratio of calcium to silicon in the synthetic magnesia which is not fired into bricks is 0.3 to 0.5, and the ratio of calcium to silicon in the high-purity seawater magnesia is 1.7 or more, and the high temperature strength thereof.
The main chemical component is MgO, and the mineral component is a pericloy crystal. The density is 3.56~3.65g/cm, the Mohs hardness is 5.5, the melting point is 2800°C, and it is significantly volatilized at 1800~2400°C. Pure periclase is colorless. With the increase of Fe2O3 and CaO content, the color changes from light to dark, and it is yellow, brown and dark brown. The grain size of the periclase increases correspondingly with the increase of calcination temperature and the prolongation of the holding time, and its hydration resistance and slag corrosion resistance are also correspondingly enhanced.
The impurities in the magnesia are derived from dolomite, talc, tremolite, quartz, etc. in the magnesite, so that the magnesia contains impurities such as SiO2, Al2O3, Fe2O3, CaO (B2O3 in the seawater magnesia). These impurities reduce the high-temperature strength of the magnesia, and the degree of influence is 1,3:11:70 in terms of Fe2O3 as compared with Cr2O3, Al2O3, and B2O3. In addition to the periclase in the magnesia, a variety of silicate minerals can be formed according to the CaO/SiO2 ratio, which have different effects on the properties of the magnesia products (Table 1).
According to the chemical composition of magnesia, the quality and phase composition of magnesia products can be predicted. When calculating the phase composition, only when the uniformity of the magnesia raw material and the calcination temperature of the material are sufficient to balance the phase composition, The phase composition will be consistent with the experimental results, and the higher the purity of the material, the more consistent it will be.
The magnesite is calcined to 700 ° C to decompose and discharge CO 2 , and calcined to 1000 ° C to form calcined magnesite (caustic magnesia), commonly known as bitter soil powder, which has loose texture and large chemical activity. Continue to raise the temperature to above 1700 ~ 1800 ° C, the magnesia grain gradually grows, the volume shrinks, the density increases, the chemical activity is greatly reduced, that is, calcined into sintered magnesia.